Harnessing the power of the placenta
Celularity’s unique technology realizes the full promise of cell therapies by harnessing the immunomodulatory and regenerative properties of the postpartum placenta.
Years of Research
Into the biological activities of placental-derived cells
Pioneering Technology Platform
To harness the power of multiple placental-derived cell types
Significant unmet Global Need
For inventory-ready, off-the-shelf allogeneic therapies
The Time has arrived
For cellular medicine to be available for everyone
Our Technology
Why use postpartum placental-derived cells?
One of the biggest challenges cell therapy developers face is the human immune system—how do you make a cell-based treatment that the patient’s immune system does not reject as foreign?
Currently approved treatments overcome this challenge by using the patient’s own cells, autologous cell therapy, an approach that is extremely expensive and difficult to scale.
At Celularity, we start with a material that normally possesses a low potential for provoking an immune response, the placenta, to develop allogeneic cell therapies that can be used without the need for matching the patient to the donor.
Then, using our proprietary technology, we convert a plentiful and normally discarded source material into potential therapeutics, with a single placenta capable of yielding 100 – 100,000+ doses, depending on the therapeutic.
Our technology makes it possible to develop cell therapies that are scalable and cost-effective, and that can accelerate preclinical development to as little as 18 – 24 months.
Our Celularity IMPACT™ Platform
Fully Integrated Product Development Cycle

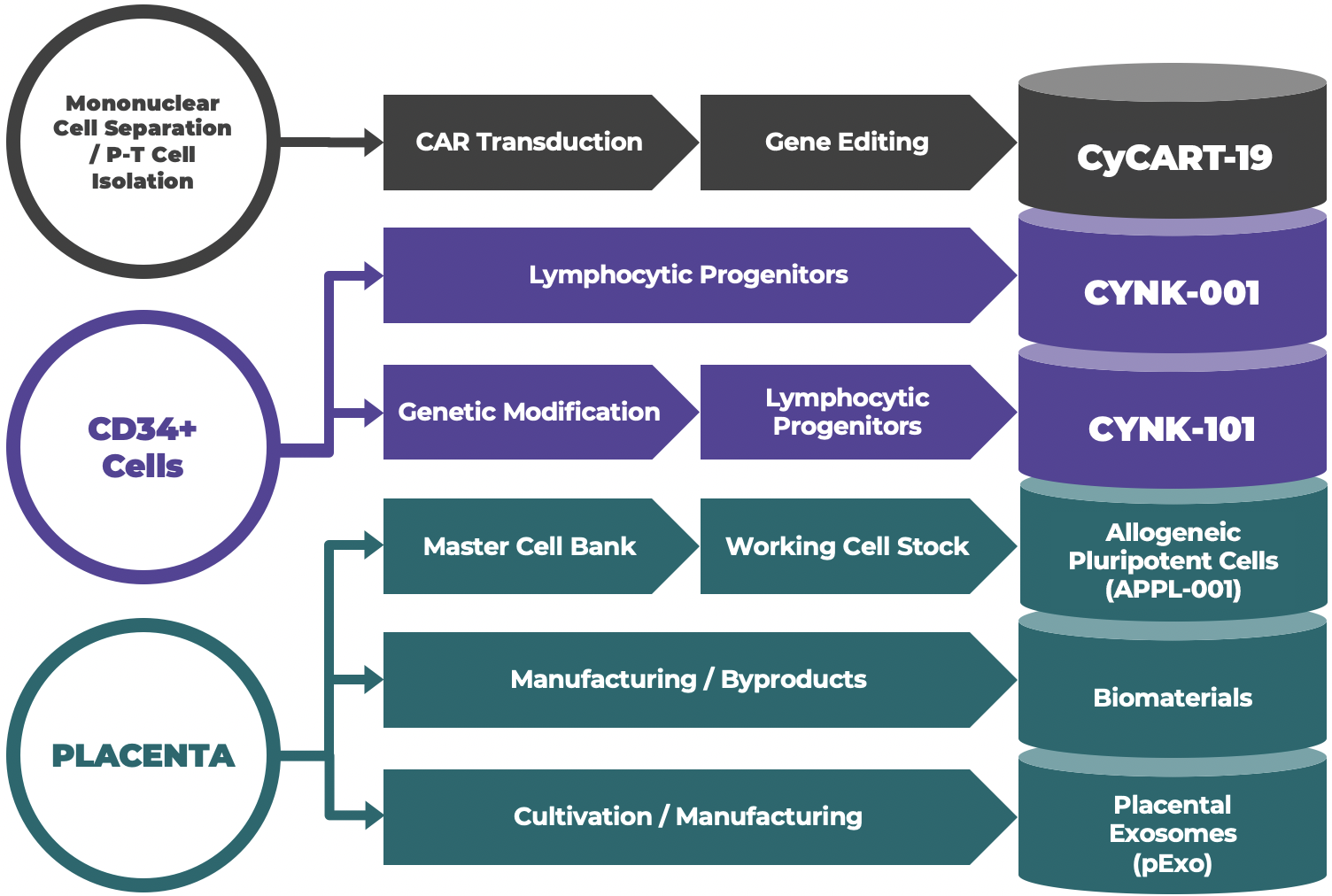
The Process is the Product
Sourcing to manufacture our full suite of products
Best In Class
Highly Scalable Biosourcing and In-house GMP Manufacturing
We obtain ethically-sourced biomaterials through an extensive, established network. Donors and biomaterials are screened and tested to verify the lack of communicable diseases, and then processed in our state-of-the-art, 150,000 square-foot, GMP/ GTP facility.
Because our manufacturing process is highly modular, production is efficient, scalable, and reproducible.
At the hospital
- Donor enrollment, qualification, selection, and informed consent
- Biomaterial collection and testing
At our manufacturing facility
- Proprietary cell harvesting and processing
- Master cell bank establishment
- Advanced cell manufacturing
Interested in joining the Celularity team of scientists, clinicians, and operators?