
Investigational Therapy for Patients Who Have Locally Advanced Unresectable or Metastatic HER2 Positive Gastric or Gastroesophageal Junction (G/GEJ) Adenocarcinoma
We are investigating CYNK-101, a novel placenta-derived cell therapy in patients with Locally Advanced Unresectable or Metastatic HER2 Positive Gastric (G) or Gastroesophageal Junction (GEJ) cancer.
The purpose of the clinical research study “CYNK-101-HER2-001” is to test the safety and effectiveness of an investigational treatment named “CYNK-101” in adults with HER2 positive G/GEJ cancer. The study plans to identify the most appropriate dose of CYNK-101 and find out if treatment with CYNK-101 improves or worsen this type of cancer.
Location

John Theurer Cancer Center at
Hackensack University Medical Center
Hackensack, NJ 07601
Investigator

Martin E. Gutierrez, MD
Hackensack University
Medical Center
About the Clinical Research Study
The CYNK-101-HER2-001 research study is recruiting individuals who have advanced cancer of the stomach or advanced cancer in the distal part of the esophagus and a genetic abnormality called human epidermal growth factor receptor 2 overexpression (HER2+). HER2 is a protein that if found in abundant quantities in your body, fuels the growth of this type of cancer.
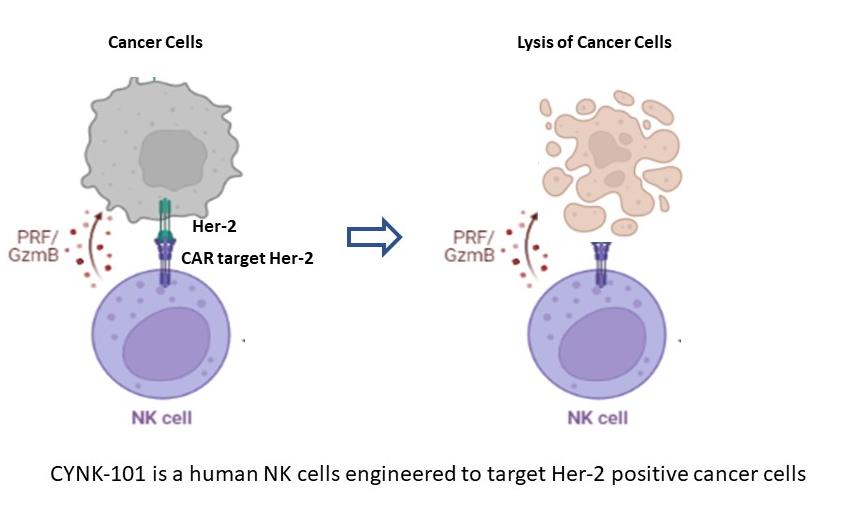
CYNK-101 is an investigational product made from human Natural Killer (NK) cells derived from placental blood cells. NK cells are engineered to target HER2 expressing cancer cells.
Celularity laboratories have conducted numerous pre-clinical tests with CYNK-101 in HER2 positive gastric cancer cell lines. The results of these pre-clinical tests have shown that CYNK-101 kills gastric cancers showing HER2 overexpression, without killing any normal healthy cells.
Length of Study
The study may take up to 24 months from the time you complete the screening evaluations until the day you receive the last study treatment. You will then have a one-year follow up period.
The study has 3 major parts:
Part 1: Screening
During this Part, you will undergo study specific tests and procedures to confirm if you are the right candidate to participate in the study. The Screening tests and procedures may take a few days during a 28-day period.
Part 2: Treatment
You may complete up to 37 visits over 24 months which will include the following:
- Induction of standard of care treatments (Pembrolizumab, Trastuzumab and Chemotherapy): Up to 126 days (approximate 4.5 months)
- Lymphodepletion treatments (Cyclophosphamide, Fludarabine and Mesna): 3 days of treatment followed by 2 days of rest (i.e., no treatments are given)
- Re-Induction of NK cells (CYNK-101) and rhIL-2: 21 days (approximate 3 weeks).
- Maintenance treatments (CYNK-101/rhIL2 and standard of care treatments) will be given depending on your response to treatment every 8 weeks: Up to 560 days (approximate 18 months)
Part 3: Follow-Up
For approximately 12 months after the last dose of CYNK-101. If you receive any treatment with CYNK-101, you will have certain specific tests to continue checking for any adverse effects of CYNK-101. You will be asked to come to the clinic every 3 months. Additional annual testing may also be required.
OVERVIEW
AGE: ≥18 years old
GENDER: Any
KEYWORDS: CYNK-101, Locally Unresectable or Metastatic Gastric or Gastroesophageal Junction (G/GEJ) adenocarcinoma, CYNK-101, Natural Killer cells, placental stem cells
TYPE: Phase I/IIa, Cellular Immunotherapy
TARGET: 52 Participants
WHO MAY PARTICIPATE?
Patients with newly diagnosed locally advanced unresectable or metastatic HER2 Positive Gastric (G) or Gastroesophageal Junction (GEJ) cancer
Patients with newly diagnosed locally advanced unresectable or metastatic HER2 Positive Gastric (G) or Gastroesophageal Junction (GEJ) cancer
Patients who are at least 18 years old
Patients who are willing to provide a biopsy
Resources: ClinicalTrials.gov NCT05207722
Have any questions or want to learn more?
Please contact the site coordinator with your questions via email.


