Emerging studies demonstrate that placental-derived NK cells hold therapeutic potential against age-related diseases by rejuvenating the immune function, and eliminating senescent cells, which accumulate with age and drive inflammation and functional decline.
– Anna Gosiewska, Sr. Vice President, Research & Development
CYNK-001
Placental-derived Natural Killer (CYNK) Cells
CYNK-001 is the only cryopreserved, allogeneic, off-the-shelf, natural killer (NK) cell therapy being developed from placental hematopoietic stem cells as a potential treatment option for various age-related diseases. NK cells are a unique class of immune cells, innately capable of targeting stressed cells including senescent cells, virally infected cells, and cancer cells.
CYNK-001 demonstrates a range of biological activities expected of NK cells, including expression of perforin and granzyme B, cytolytic activity against hematological tumors and solid tumor cell lines, and secretion of immunomodulatory cytokines such as IFN-γ in the presence of tumor cells. CYNK-001 cells express NKG2D and CD94, as well as NK activating receptors DNAM1, NKp30, NKp46, and NKp44.
Preclinical studies show that CYNK-001 displays cell killing activity against MM cells, AML cells, GBM cells, and SARS-CoV-2-infected cells in vitro.
We have clinical experience in the following indications:
Acute Myeloid Leukemia
NCT04310592
Program Objective
Achieve durable response for newly diagnosed AML patients in frontline setting
Target Population
Newly diagnosed AML patients
Global Incidence in 2019
40,000 new AML cases
Glioblastoma Multiforme
NCT04489420
Program Objective
Stabilize disease to prevent progression
Target Population
GBM patients
Global Incidence in 2019
37,000 new GBM patients
Multiple Myeloma
NCT04309084
Program Objective
Achieve durable response for MM patients eligible for ASCT in frontline setting
Target Population
Newly diagnosed MM patients following stem cell transplant
Global Incidence in 2019
70,000 new MM patients
COVID-19
NCT04365101
Program Objective
Limiting viral replication and eliminating infected cells
Target Population
COVID-19 patients
Global Incidence in 2020
4.7 Million USA Confirmed Cases in 2020
CYNK-101
Genetically-modified Placental NK Cells (CYNK)
We are also developing pipeline programs dedicated to augmenting NK cells’ natural mechanisms to recognize and eradicate stressed cells including senescent cells, virally infected cells or cancer, through various genetic modification approaches.
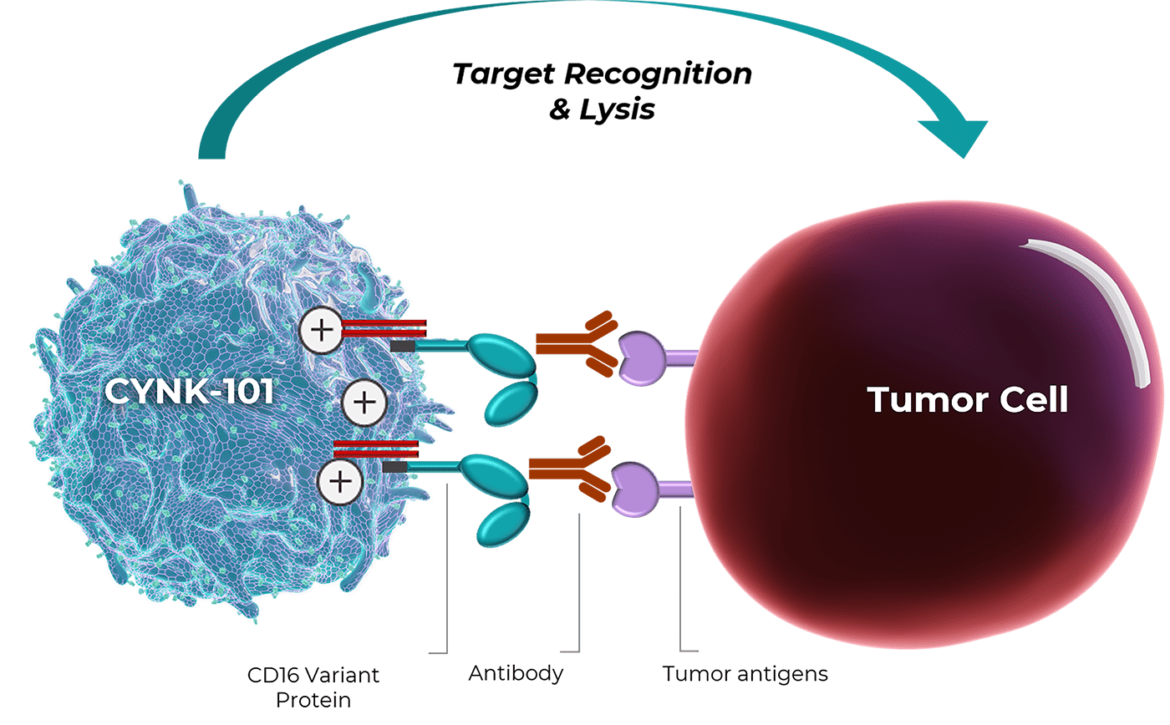
We have developed CYNK-101 as a platform for combination treatment for multiple cancer indications. CYNK-101 is manufactured from a specific type of immune cell, NK cells, that we extract from postpartum placentas and then genetically engineer to express a high-affinity cleavage-resistant Fc receptor CD16 to increase cell killing activity when introduced together with an antibody-based anti-cancer treatment.
Preclinical studies show that CYNK-101 plus an antibody therapeutic displays cell killing activity against lymphoma cells in vitro. We expect CYNK-101 to work in vivo by augmenting antibody-dependent cytotoxicity (ADCC), increasing the efficacy of already-approved antibody therapeutics.
We are also engineering CYNKs to directly recognize tumor cells even in the absence of the appropriate signals by expressing chimeric antigen receptors (CARs) on their surface. This enhances anti-tumor activity even when tumors express high levels of inhibitory ligands.
CYNK-101 augments ADCC against tumor targets in combination with monoclonal antibodies
The FDA clearance of our IND validates the versatility of our allogeneic, off-the-shelf, placental-derived NK cell therapy platform to generate novel clinical candidates against a broad range of devastating cancers. This IND represents a significant step toward a potential immunotherapy option that is more accessible and tolerable to patients with glioblastoma multiforme.
– Robert J Hariri, MD, PhD
RESOURCES
Frontiers in Immunology
Senescence, NK cells, and cancer: navigating the crossroads of aging and disease.
61ST ASH ANNUAL MEETING, 2019, POSTER SESSION
Immune Monitoring of CD34+ Placental Cell Derived Natural Killer Cell Therapy (PNK-007) in Phase I Study of Multiple Myeloma
61ST ASH ANNUAL MEETING, 2019, POSTER SESSION
Results of a Phase I Study of PNK-007, Allogeneic, Off the Shelf NK cell, Post Autologous Transplant in Multiple Myeloma
61ST ASH ANNUAL MEETING, 2019, POSTER SESSION
Engineering High Affinity and Cleavage Resistant CD16 to Augment ADCC of Placental Hematopoietic Stem Cells Derived Natural Killer Cells
61ST ASH ANNUAL MEETING, 2019, POSTER SESSION
Development of CD38 CAR Engineered Human Placental Hematopoietic Stem Cell Derived Natural Killer Cells (PNK-CAR38) As Allogeneic Cancer Immunotherapy
34TH SITC ANNUAL MEETING, 2019, POSTER SESSION
Mechanisms underlying human placental CD34+ cell-derived natural killer cell cytotoxicity against glioblastoma
24TH SNO ANNUAL MEETING, 2019, POSTER SESSION
Human Placental Hematopoietic Stem Cell Derived Natural Killer Cells for Glioblastoma Immunotherapy
110TH AACR ANNUAL MEETING, 2019, POSTER SESSION
Immune monitoring of PNK-007, an allogeneic, off the shelf NK cell in a Phase I study of acute myeloid leukemia
110TH AACR ANNUAL MEETING, 2019, POSTER SESSION
A Phase I Study of PNK-007, an Allogeneic, Off the Shelf NK cell in relapsed/refractory Acute Myeloid Leukemia
24TH EHA ANNUAL MEETING, 2019, POSTER SESSION
Safety and Tolerability of Allogeneic, Off-the-Shelf Placental Natural Killer Cells (PNK-007) in Phase 1 Multiple Myeloma and Acute Myeloid Leukemia
33TH SITC ANNUAL MEETING, 2018, POSTER SESSION
Potent ex vivo Expanded, Human Placental CD34+-derived Natural Killer Cells for Glioblastoma Immunotherapy
NK2018, POSTER SESSION
Potent ex vivo Expanded, Human CD34+ Cord Blood-derived Natural Killer Cells for Cancer Immunotherapy